How Are Positive And Negative Ions Formed
Olivia Luz
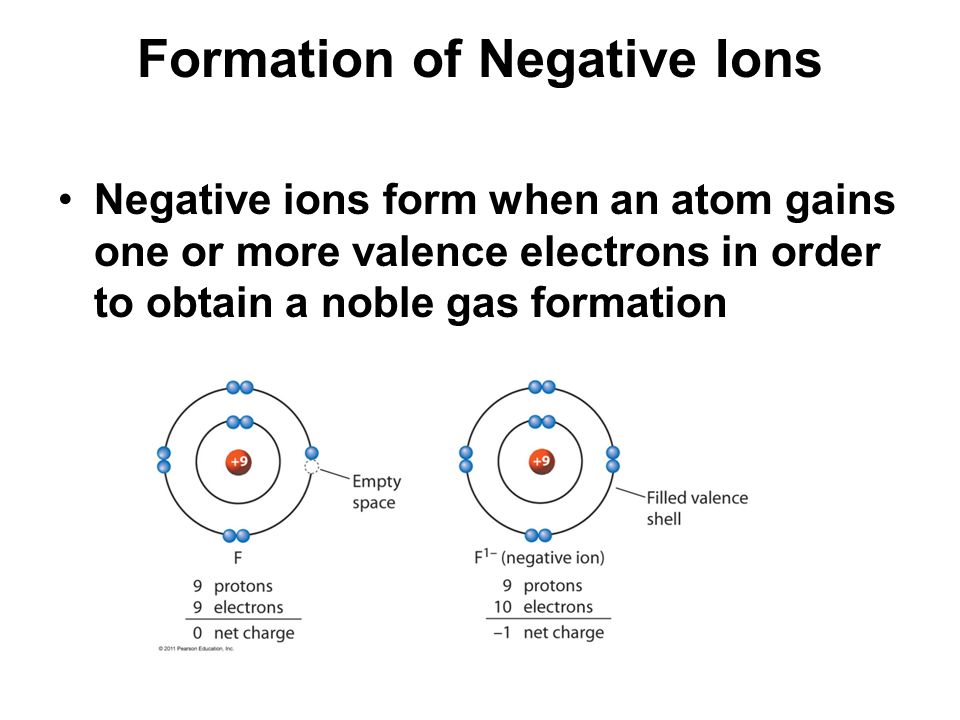
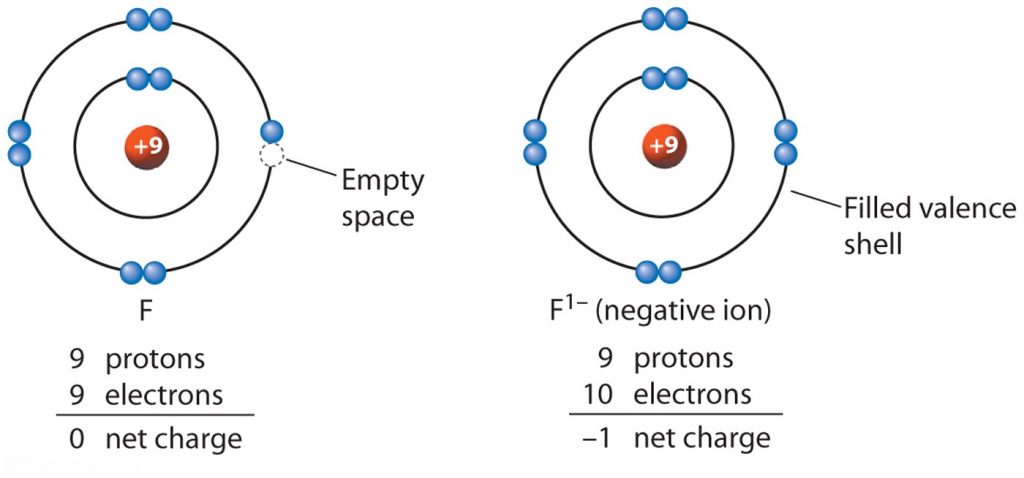
Negative ions also known scientifically as anions are the opposite of positive ions and they have the opposite effect.
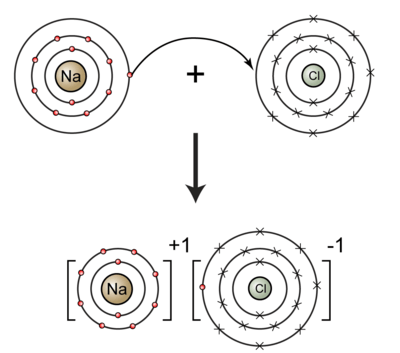
Cations and anions cations positively charged ions and anions negatively charged ions are formed when a metal loses electrons and a nonmetal gains those electrons. Each positive ion has negative ions as its nearest neighbours and each negative ion has positive ions as its near neighbors. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound such as sodium chloride. The definition of a negative ion is an electrically charged atom or cluster of atoms formed by gaining one or more electrons.
Ions form when atoms lose or gain electrons to obtain a full outer shell. An ion is an atom or group of atoms with a positive or negative charge. How are positive and negative ions formed. What are positive and negative ions and how are they formed.
Metal atoms lose electrons to form positively. Positive and negative ions are formed by gaining or losing electrons from neutral atoms. Metallic elements produce positively charged ions by losing electrons while nonmetallic elements produce negatively charged ions by gaining electrons. Electrons in an atom are surrounded by a positively charged electron cloud and a negatively charged ion cloud.
RELATED ARTICLE :
With the electrons spinning at a specific rate these forces create charge separation between the atoms. Inside the atom of every element are sub particles. The number of protons in the atom does not change but the extra electrons gives it a negative charge. An ionic bond is formed by ions of opposite charges in a regular lattice.Source : pinterest.com